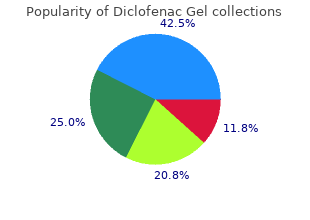
Diclofenac Gel
"Order diclofenac gel 20 gm without prescription, arthritis in neck and back".
By: D. Gambal, M.A., M.D., M.P.H.
Medical Instructor, University of Maryland School of Medicine
It is possible therefore that the induction treatment sequence may not be appropriate for all patients arthritis definition in hindi generic diclofenac gel 20 gm overnight delivery. If withdrawal from treatment results in flare of disease rheumatoid arthritis diet recipes discount diclofenac gel, the cost effectiveness of all comparators may have been overestimated in the model rheumatoid arthritis definition of remission order diclofenac gel 20 gm with mastercard. A model relevant to the paediatric population could not be constructed due to paucity of clinical data. In reality, patients may experience greater severities of relapse which may necessitate treatment options different to those captured in the model. Similarly, other extents of ulcerative colitis such as proctitis have not been addressed and as such treatment options used in the model may not be applicable. As immunomodulators are often started in addition to corticosteroids for the induction of remission to allow the tapering of corticosteroids and their continuation as a maintenance treatment, studies that have used this treatment regimen and randomised the patients at the point of induction have also been included but analysed separately. There is a risk of bias associated with this method due to not all of the patient�s entering remission or taking different times to enter remission and this has been taken into account when interpreting the results of these studies. For these studies, relapse figures are calculated as those who relapse after having remission induced. There were only 2 studies identified that matched our inclusion criteria which looked at the use of azathioprine for the maintenance of remission. The review included 6 studies which looked at the following comparisons: � Azathioprine versus placebo � Azathioprine versus 6-mercaptopurine � 6-mercaptopurine versus methotrexate the Cochrane review concluded that azathioprine is clinically more effective at maintaining remission compared to placebo, and that azathioprine or 6-mercaptopurine could be used for maintenance treatment in people who have failed or cannot tolerate mesalazine or sulphasalazine and for those who require repeated courses of corticosteroids. The Cochrane review was excluded because it differed from the clinical review protocol in terms of the methods of analysis; the clinical review used hazard ratios in preference to relative risk ratios to take account of the time horizon and studies where the patients are randomised at the point of induction are separated from those in which patients have been in remission for a period of time. Combinations of azathioprine and an aminosalicylates were also not included in the Cochrane review. However, all of the studies included in the Cochrane review appear in the clinical review. The Cochrane review concluded that there was insufficient evidence to recommend the use of methotrexate to maintain remission in patients with ulcerative colitis. Although the Cochrane review has been excluded as it did not report relapse as an outcome, only maintenance of remission, the same study has been included in the clinical review. However, it is noted that there were significant differences in the number of transient leukopenia events (5 in the azathioprine group and 12 in the azathioprine and olsalazine group). These were transient leukopenia (n=1) and migraine (n=1) in the methotrexate group and a severe rash (n=1) in the placebo group. In addition to drug costs, costs would be incurred in the due to monitoring blood levels to ensure therapeutic response. To maintain remission after a mild to moderate inflammatory National Clinical Guideline Centre, 2013. To maintain remission after a single episode of acute severe ulcerative colitis: nn nn � consider oral azathioprine or oral mercaptopurine � consider oral aminosalicylates in people who cannot tolerate or who decline azathioprine and/or mercaptopurine, or in whom azathioprine and/or mercaptopurine are contraindicated. These were considered the critical outcomes in making decisions about the maintenance of remission. Topical preparations are thought not to extend to the colon and are unsuitable for treating people with left sided or extensive disease. Proctitis/proctosigmoiditis the evidence for recommendation 27 is limited and is reflected in the strength of the recommendation. Intermittent topical treatment in the trials is defined as the first seven days of 48 3 the month (D�Albaso), twice weekly (Andreoli) and every third night 133 (Mantzaris). The recommendation makes it clear that this may not be as effective a treatment option but that it may be better than no treatment. In relation to combination treatment (oral plus topical treatment) the evidence was limited. Children and young people Recommendation 29 is specific to children and young people. It is important for this population to have access to this treatment option despite the absence of evidence and this has been reflected in recommendation 30. However, it is possible that cost savings could be made if a suppository is used over an enema and if intermittent dosing is chosen over daily dosing. This was supported by two studies which showed that there are increased drug costs associated with combination therapy; however combination treatment may be better for maintaining remission. There were a number of limitations with the studies as outlined in the economic evidence profile. It is plausible however, that if patients are successfully maintained in remission, downstream costs due to additional drug use and hospitalisations could be reduced.
Equally important is knowing how rheumatoid arthritis in children's feet order diclofenac gel 20gm with visa, when arthritis care of texas order diclofenac gel 20 gm online, where and why to yoga arthritis pain buy discount diclofenac gel on-line ask about it, to acknowledge it in a way that feels comfortable and genuine, and is appropriate in the current circumstances. There are times when asking about trauma is not appropriate, and/or the provider must be mindful of guiding the conversation in a way that doesn�t lead the client to feel overwhelmed. Adopting universal precautions would suggest that we relate to everyone based on an assumption that they have had traumatic experiences. The matter of universal screening is another important issue for which each organization must establish its own protocol. We can ask people about whether they have had traumatic experiences without encouraging them to describe these events in detail. In doing so, however, it is important that people know why the questions are being asked and to understand that they do not need to answer them. Following are a series of scenarios outlining how to appropriately ask about trauma and respond in different circumstances. How do I ask about trauma when a person doesn�t come out and say it, but gives other indications that they are having diffcultiesfi Some examples include �What are your thoughts about what these feelings might be connected tofi They are very emotional, and you feel quite moved and saddened by their experiences. You take your time to decide how you would like to address this because you want to help them feel accepted and comfortable. For example, �Sounds like you are going through a hard time, and that makes sense given what you�ve already gone through. What I meant to say was, that was a terrible experience, and I�m so glad you were able to fnd safety. What if someone discloses trauma and they want to tell me all about it, but it�s not my role or responsibility to be a counsellorfi She goes on at length about the situation, asks for your advice, and says that she feels she needs to work on the impacts she is only now acknowledging. A more appropriate response is to refer them to the service that is right for them and their situation, and that they are willing to use. For example, you could say, �This is a hard time for you, and I thank you for sharing this with me. Sounds like you have a lot to talk about and I�m wondering if counselling is an option for you right nowfi You ask him if his father abused him, and his reply is, �Yeah, he was really mean and he�d let you know with his fsts when he was angry. For example, �You described physical abuse by your dad, and I know abuse can often be sexual, too. For example, �Abuse is never the fault of the child; you were in a situation where you had no choices. Sexual abuse cannot make you gay because it is used as a weapon, but society sure seems to send us that message. For example, �I know a lot of guys who were beat up as kids; good thing you�ve moved on from that now. She states that she�s been bombarded with memories and fashbacks recently, has missed work, is crying a lot, and isn�t really feeling she�s in reality. This individual is not physically or mentally able to function properly, so asking about the trauma may exacerbate the situation by adding to her inability to cope. Do you have time to talk about the memories and how they are impacting you 118 nowfi Do I need to get all the details of the trauma in order to understand where the individual is coming from and for them to healfi Just asking about the feelings and impacts of the trauma is all that is necessary to encourage healing and recovery. For example, �Can you please start from the beginning and tell me in detail about your experiences that are still painfulfi When you ask him about his depression, he provides little information and seems uncomfortable, like he doesn�t want to be there, even though he came voluntarily. Understand what his �normal� way of communicating is and place your work with him in that context. I heard someone call my name, and it was my co-worker running after me with my purse.
Enzalutamide versus abiraterone acetate for the treatment of men with metastatic castration-resistant prostate cancer arthritis itchy back order diclofenac gel online now. Prostate-specific antigen: an evolving role in diagnosis arthritis relief in thumb buy cheap diclofenac gel on-line, monitoring arthritis virus purchase diclofenac gel 20gm line, and treatment evaluation in prostate cancer. Second-line chemotherapy with docetaxel for prostate-specific antigen relapse in men with hormone refractory prostate cancer previously treated with docetaxel based chemotherapy. Randomized trial of short versus long-course radiotherapy for palliation of painful bone metastases. A Multicenter Randomized Trial of Ibandronate Compared With Single-Dose Radiotherapy for Localized Metastatic Bone Pain in Prostate Cancer. Percutaneous vertebral augmentation: an elevation in adjacent-level fracture risk in kyphoplasty as compared with vertebroplasty. Time of survival and quality of life of the patients operatively treated due to pathological fractures due to bone metastases. Segmental polymethylmethacrylate-augmented pedicle screw fixation in patients with bone softening caused by osteoporosis and metastatic tumor involvement: a clinical evaluation. Functional and oncological outcome of acetabular reconstruction for the treatment of metastatic disease. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone refractory metastatic prostate carcinoma. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Guidance on the use of bisphosphonates in solid tumours: recommendations of an international expert panel. Definitions of biochemical failure that best predict clinical failure in patients with prostate cancer treated with external beam radiation alone: a multi-institutional pooled analysis. Defining biochemical recurrence of prostate cancer after radical prostatectomy: a proposal for a standardized definition. Management of prostate-specific antigen relapse in prostate cancer: a European Consensus. Ultrasensitive serum prostate specific antigen nadir accurately predicts the risk of early relapse after radical prostatectomy. Prognostic implications of an undetectable ultrasensitive prostate-specific antigen level after radical prostatectomy. Prostate specific antigen in the diagnosis and treatment of adenocarcinoma of the prostate. Evaluation of serum prostate-specific antigen velocity after radical prostatectomy to distinguish local recurrence from distant metastases. The incidence of prostate cancer progression with undetectable serum prostate specific antigen in a series of 394 radical prostatectomies. Prostate specific antigen after radiotherapy for prostate cancer: a reevaluation of long-term biochemical control and the kinetics of recurrence in patients treated at Stanford University. Digital rectal examination is no longer necessary in the routine follow-up of men with undetectable prostate specific antigen after radical prostatectomy: the implications for follow up. Prostate specific antigen: a prognostic marker of survival in good prognosis metastatic prostate cancerfi Prostate-specific antigen nadir and cancer-specific mortality following hormonal therapy for prostate-specific antigen failure. The prognostic value of hemoglobin change after initiating androgen-deprivation therapy for newly diagnosed metastatic prostate cancer: A multivariate analysis of Southwest Oncology Group Study 8894. Prostate specific antigen and bone scan correlation in the staging and monitoring of patients with prostatic cancer. Pelvic lymph node dissection during robot-assisted radical prostatectomy: efficacy, limitations, and complications-a systematic review of the literature. Complications and other surgical outcomes associated with extended pelvic lymphadenectomy in men with localized prostate cancer.
Syndromes
- Activated charcoal
- Problems attaining or maintaining an erection (impotence)
- Deciding to breastfeed
- Rib cage
- No improvement after 3 days of home treatment
- Burns
- Long-term, unexplained tearing
- Fluids by IV
During audits/inspections the auditors/inspectors may copy relevant parts of the medical records arthritis gene order diclofenac gel 20gm on line. No personal identification apart from the screening/randomisation number will appear on these copies arthritis symptoms in fingers buy diclofenac gel master card. If deviations occur arthritis relief without nsaids discount 20 gm diclofenac gel with mastercard, the Investigator must inform the Monitor, and the implications of the deviation must be reviewed and discussed. In addition, a set of deviations must be accompanied by a description of the deviation, the relevant dates (start and stop), and the action taken. Deviation reports and supporting documentation must be kept in the Investigator�s File and in the Trial Master File. Should this become necessary, the procedures will be agreed upon after consultation between the two parties. In terminating the trial, the Sponsor and the Investigator will ensure that adequate consideration is given to the protection of the best interests of the patients. In addition, the Sponsor reserves the right to terminate the participation of individual trial sites. Conditions that may warrant termination include, but are not limited to, insufficient adherence to protocol requirements and failure to enter patients at an acceptable rate. The Investigator and any other persons involved in the trial will protect the confidentiality of this proprietary information belonging to the Sponsor. In a multi-site trial based on the collaboration of many sites, any publication of results must acknowledge all sites. Results from multi-site trials must be reported in entirety in a responsible and coherent manner and results from subsets should not be published in advance or without clear reference to the primary publication of the entire trial. The Sponsor reserves the right to be last author(s) in all publications related to this trial, with a maximum of three employees of the Sponsor per publication. In the event of any disagreement in the content of any publication, both the Investigator�s and the Sponsor�s opinion will be fairly and sufficiently represented in the publication. If the Investigator wishes to independently publish/present any results from the trial, the draft manuscript/presentation must be submitted in writing to the Sponsor for comment prior to submission. This statement does not give the Sponsor any editorial rights over the content of a publication, other than to restrict the disclosure of the Sponsor�s intellectual property. If the matter considered for publication is deemed patentable by the Sponsor, scientific publication will not be allowed until after a filed patent application is published. Under such conditions the publication will be modified or delayed at the Investigator�s discretion, to allow sufficient time for the Sponsor to seek patent protection of the invention. This policy requires that all clinical trials be registered in a public, clinical trials registry. Thus, it is the responsibility of the Sponsor to register the trial in appropriate registries. All ethical and regulatory approvals must be available before a patient is exposed to any trial-related procedure, including screening tests for eligibility. In addition, a summary of the clinical trial report will be provided when available and within one year of trial completion (defined as Last Patient Last Visit). The patient will receive a copy of the patient information and his signed consent. The Investigator will obtain a freely given written consent from each patient after an appropriate explanation of the aims, methods, anticipated benefits, potential hazards, and any other aspects of the trial which are relevant to the patient�s decision to participate. The trial patient must be given ample time to consider participation in the trial, before the consent is obtained. The informed consent form must be signed and dated by the patient before he is exposed to any trial-related procedure, including screening tests for eligibility. The Investigator will explain that the patients are completely free to refuse to enter the trial or to withdraw from it at any time, without any consequences for their further care and without the need to justify their decision. The trial patients will be informed about this new information and re-consent will be obtained. Each patient will be informed that the monitor(s), quality assurance auditor(s) mandated by the Sponsor, or regulatory authority inspector(s), in accordance with applicable regulatory requirements, may review his/her source records and data. The study site should plan on retaining such documents for approximately 15 years after study completion. These documents should be retained for a longer period if required by the applicable regulatory requirements or the hospital, institution, or private practice in which the study is being conducted.
Diclofenac gel 20 gm. USE OF MEDICINES ARTHRITIS HEALTH EDUCATION INFECTION CONTROL (ICSP) URDU / HINDI.